The reaction of bromine gas with chlorine gas, shown here, has a Kp value of 7.20. If a closed vessel was charged with the two reactants, each at an initial partial pressure of 0.500 atm, what would be the equilibrium partial pressure of BrCl(g) ? Br2(g) + Cl2(g) 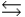 2BrCl(g)
2BrCl(g)
A) 1.9 atm
B) 1.4 atm
C) 0.84 atm
D) 0.29 atm
E) 0.57 atm
Correct Answer:
Verified
Q53: The reaction of bromine gas with chlorine
Q53: What is the difference between
Q54: In a simple equilibrium A + B
Q56: A reaction X + 2Y
Q57: When can an x be ignored in
Q59: Which of the following occurs when products
Q60: To solve an equation of the form
Q65: If the temperature of an endothermic reaction
Q65: Which of the following must be
Q76: Increasing the temperature of an exothermic reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents