The sulfide ion, S2-, reacts with water as a weak base: S2- + H2O 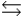 SH- + OH- K = 1.0
SH- + OH- K = 1.0
If sodium sulfide were dissolved in water to make a solution of 0.50 M, what would be the resulting concentration of OH-?
A) 0.50 M
B) 0.37 M
C) 0.63 M
D) 1.0 M
E) 0.19 M
Correct Answer:
Verified
Q37: For the chemical equilibrium aA + bB
Q39: For the chemical equilibrium aA +bB
Q40: The equilibrium constant for the formation of
Q43: Cylinders of NO gas may contain small
Q44: Which of the following relationships are
Q45: What happens to the equilibrium between NO2(g)
Q46: The gas-phase equilibrium of the oxidation
Q47: A student determined the equilibrium concentration in
Q57: Lime is obtained _
A) by heating
Q72: Which of the following is true regarding
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents