A sealed tube containing an equilibrium mixture of NO2(g) (brown) and N2O4(g) (colorless) is subjected to a variety of temperatures. For the equilibrium reaction shown, the values of the H 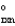 and S
and S 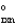 are also shown. What is observed in the tube with increasing temperature? 2NO2(g)
are also shown. What is observed in the tube with increasing temperature? 2NO2(g) 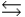 N2O4(g) H = -58.02 kJ/mol, S°=-176.5 J/mol · K
N2O4(g) H = -58.02 kJ/mol, S°=-176.5 J/mol · K
A) At higher temperatures, the gas in the tube has a darker brown color.
B) At higher temperatures, the gas in the tube has a lighter brown color.
C) At higher temperatures, the gas in the tube is colorless.
D) The color does not change with temperature.
E) At higher temperatures, the gas turns green.
Correct Answer:
Verified
Q80: Using the thermodynamic data below, determine
Q83: For a chemical reaction at equilibrium, which
Q84: Chemical equilibrium arises from the _ of
Q86: Which of the listed perturbations will change
Q87: Hydrofluoric acid, HF, dissociates in water according
Q88: A chemist is planning to study
Q89: A reaction is run under conditions where
Q90: Consider the reaction of nitrogen and hydrogen
Q103: Students often write expressions for the equilibrium
Q108: How and why are the expressions for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents