Gaseous carbon monoxide and hydrogen gas can be used to prepare methanol (CH3OH). Under equilibrium conditions at 427°C, the partial pressures of the components are as follows.
PCO = 1.44 atm 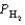 = 4.25 atm
= 4.25 atm 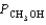 = 2.30 atm
= 2.30 atm
What is the value of the equilibrium constant for the reaction in which one mole of methanol is produced?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q87: For the reaction of hydrogen and nitrogen
Q96: The
Q97: Write an expression for the equilibrium constant
Q101: If Q for a reaction is greater
Q101: Which of the listed perturbations would change
Q104: Calculate the equilibrium constant at 500
Q111: Given the following two measurements of
Q149: Nitrogen monoxide molecules can react to
Q153: Consider a reaction at a point in
Q156: Values for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents