The G° of atomic oxygen is 230.1 kJ/mol, and S° is 118 J/mol · K. In the stratosphere, however, the product gas-phase oxygen predominates. Why? O2(g) 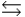 2O(g)
2O(g)
A) Photochemical energy of UV light drives the equilibrium forward.
B) Low pressures drive the equilibrium forward.
C) Low partial pressures make the reverse rate very small.
D) All the above are correct.
Correct Answer:
Verified
Q82: For the reaction of hydrogen and nitrogen
Q87: For the reaction of hydrogen and nitrogen
Q92: The values of
Q93: For a reaction to be spontaneous, the
Q97: Write an expression for the equilibrium constant
Q100: Gaseous carbon monoxide and hydrogen gas can
Q101: If Q for a reaction is greater
Q101: Which of the listed perturbations would change
Q111: One mole of solid ammonium carbamate (NH4CO2NH2)
Q111: Given the following two measurements of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents