The values of H°rxn and S°rxn are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature? 2NO2(g) 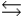 N2O4(g) H = -58.02 kJ/mol, S°=-176.5 J/mol · K
N2O4(g) H = -58.02 kJ/mol, S°=-176.5 J/mol · K
A) The negative value of H is dominant at all temperatures.
B) The negative value of S° is dominant at all temperatures.
C) The negative value of S° is combined with the small value of T.
D) The negative value of H is combined with the small value of T.
Correct Answer:
Verified
Q82: For the reaction of hydrogen and nitrogen
Q87: Hydrofluoric acid, HF, dissociates in water according
Q87: For the reaction of hydrogen and nitrogen
Q88: A chemist is planning to study
Q89: A reaction is run under conditions where
Q90: Consider the reaction of nitrogen and hydrogen
Q93: For a reaction to be spontaneous, the
Q96: The
Q97: Write an expression for the equilibrium constant
Q111: One mole of solid ammonium carbamate (NH4CO2NH2)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents