At the point marked with a dot on the phase diagram, the solid will __________ 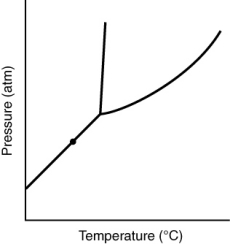
A) be indistinguishable from the gas.
B) boil.
C) melt.
D) sublime.
E) liquify.
Correct Answer:
Verified
Q14: A hydration sphere forms around an ion
Q53: The relative energies (strengths) of the intermolecular
Q54: The relative energies (strengths) of the intermolecular
Q55: At the critical point, _
A) all the
Q56: Which of the following substances has a
Q59: Consider the phase diagram for a substance
Q61: Which one of the following substances would
Q62: The temperature at point b in the
Q67: A phase diagram shows the states of
Q118: Water forms a concave meniscus in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents