Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds so any missing bonds are implicitly bonds to hydrogen atoms.
A) 
B) 
C) 
D) 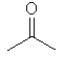
E) 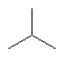
Correct Answer:
Verified
Q14: A hydration sphere forms around an ion
Q56: Which of the following substances has a
Q58: At the point marked with a dot
Q59: Consider the phase diagram for a substance
Q62: The temperature at point b in the
Q64: Which statement about the phase diagram below
Q65: The relative energies (strengths) of the intermolecular
Q66: Point d in the phase diagram below
Q67: A phase diagram shows the states of
Q118: Water forms a concave meniscus in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents