Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below. Explain why you think this molecule is planar or nonplanar. 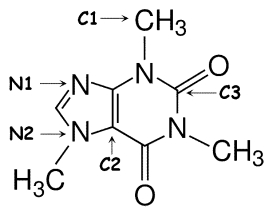
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q78: Oxygen has two common molecular anions: peroxide
Q80: Which of the following molecules is paramagnetic?
A)
Q82: Predict the following three bond angles and
Q84: Use the relative energies of the
Q86: A Lewis structure of aspirin without the
Q87: Identify the molecular structure of the nitrate
Q108: Of the following molecules (O3, SCl2, SO2,
Q113: Predict the bond order of the NO+
Q129: Methyl thiocyanate (CH3SCN) is used as an
Q177: For which one of the following molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents