Use the relative energies of the molecular orbitals given below, which are derived from the n = 5 atomic orbitals, to determine the bond orders in I2 , 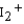 , and
, and 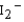 , and identify the species that is predicted to be the least stable. The valence molecular orbitals in order of increasing energy are * * *.
, and identify the species that is predicted to be the least stable. The valence molecular orbitals in order of increasing energy are * * *.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q80: Which of the following molecules is paramagnetic?
A)
Q82: Predict the following three bond angles and
Q83: Identify the hybridization of atomic orbitals for
Q86: A Lewis structure of aspirin without the
Q87: Identify the molecular structure of the nitrate
Q97: Which one of the following statements
Q108: Of the following molecules (O3, SCl2, SO2,
Q113: Predict the bond order of the NO+
Q129: Methyl thiocyanate (CH3SCN) is used as an
Q177: For which one of the following molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents