How many orbitals exist for the quantum numbers n = 4, 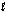 = 1?
= 1?
A) 1
B) 2
C) 3
D) 4
E) 7
Correct Answer:
Verified
Q46: Determine the wavelength of the line in
Q57: Which of the following objects, all moving
Q61: Which combination of quantum numbers is possible
Q62: Which of the following represents an s
Q64: What are the principal and angular momentum
Q67: Which subshell only has five orbitals?
A)
Q68: Which of the following orbitals is the
Q71: The mathematical description of an electron as
Q93: The size of a typical atomic orbital
Q101: Which of the following is the ground-state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents