What are the principal and angular momentum quantum numbers for a 4f orbital?
A) n = 4, 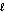 = 2
= 2
B) n = 4, 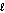 = 3
= 3
C) n = 4, 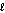 = 4
= 4
D) n = 3, 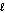 = 4
= 4
E) n = 4, 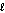 = 1
= 1
Correct Answer:
Verified
Q46: Determine the wavelength of the line in
Q61: Which combination of quantum numbers is possible
Q62: Which of the following represents an s
Q63: How many orbitals exist for the quantum
Q67: Which subshell only has five orbitals?
A)
Q68: Which of the following orbitals is the
Q71: The mathematical description of an electron as
Q93: The size of a typical atomic orbital
Q101: Which of the following is the ground-state
Q102: The atomic radius of germanium (Z =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents