The combustion of butane (C4H10) forms carbon dioxide and water. What is the stoichiometric coefficient for oxygen in the balanced equation when 1 mol of butane undergoes combustion?
A) 9
B) 13
C) 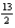
D) 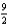
E) 5
Correct Answer:
Verified
Q26: Sulfur dioxide from coal-fired power plants combines
Q31: A balanced chemical reaction equation is to
Q37: A fuel cell works on the
Q42: Hydrogen peroxide (H2O2) decomposes catalytically in
Q43: A gas is produced when fruit ripens;
Q57: The combustion of ethanol (CH3CH2OH, 46.1 g/mol)
Q58: Glucose (C6H12O6) is oxidized by molecular oxygen
Q61: An iron ore, magnetite, contains only iron
Q64: Baking ammonia or ammonium bicarbonate (NH4HCO3, 79.1
Q64: The average adult exhales about 1.0 kg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents