Hydrogen peroxide (H2O2) decomposes catalytically in the presence of metal ions and enzymes called peroxidases. You may have noticed this reaction occurring if you use a drugstore solution of hydrogen peroxide as a mouthwash or antiseptic. If a 250 mL bottle of hydrogen peroxide solution containing 3.0% H2O2 (by mass) completely decomposes, how many liters of oxygen gas would it generate? Assume that 1 mol of gas occupies a volume of 22.4 L and that the density of the solution is 1.0 g/mL. The reaction is 2H2O2(aq) 2H2O( 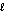 ) + O2(g)
) + O2(g)
A) 2.5 L
B) 4.9 L
C) 9.9 L
D) 7.4 L
E) 0.22 L
Correct Answer:
Verified
Q26: Sulfur dioxide from coal-fired power plants combines
Q31: A balanced chemical reaction equation is to
Q37: A fuel cell works on the
Q40: The combustion of butane (C4H10) forms carbon
Q43: A gas is produced when fruit ripens;
Q54: Baking soda (NaHCO3, 84.0 g/mol) requires
Q61: An iron ore, magnetite, contains only iron
Q64: Baking ammonia or ammonium bicarbonate (NH4HCO3, 79.1
Q64: The average adult exhales about 1.0 kg
Q92: When are an empirical formula and a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents