Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced? 2H2O ( 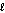 ) 2H2(g) + O2(g)
) 2H2(g) + O2(g)
A) 17.8 g
B) 8.9 g
C) 35.6 g
D) 16.0 g
E) 10.0 g
Correct Answer:
Verified
Q57: The space shuttle uses liquid hydrogen
Q60: Copper sulfate is a blue solid
Q63: Copper was the first metal to be
Q63: A careless student who was instructed
Q72: What mass of phosphoric acid (H3PO4, 98.0
Q82: Caffeine has an elemental analysis of 49.48%
Q83: A hydrocarbon molecule contains carbon and hydrogen
Q95: Combustion analysis of an organic compound to
Q101: A copper ore consisting of 12.5% copper(II)
Q104: U.S. Lime & Minerals is a company
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents