The space shuttle uses liquid hydrogen and liquid oxygen to produce thrust for liftoff in the following reaction. If the liquid oxygen supply is set to provide 420 kg/s during a launch, what does the rate of liquid hydrogen supply need to be in kilograms per second? 2H2(l) + O2( 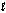 ) 2H2O(g)
) 2H2O(g)
A) 840 kg/s
B) 210 kg/s
C) 106 kg/s
D) 52.9 kg/s
E) 63.4 kg/s
Correct Answer:
Verified
Q53: The most abundant metal in Earth's crust
Q60: Copper sulfate is a blue solid
Q60: Water can be separated into its
Q63: Copper was the first metal to be
Q67: A compound known to contain platinum, nitrogen,
Q72: What mass of phosphoric acid (H3PO4, 98.0
Q82: Caffeine has an elemental analysis of 49.48%
Q83: A hydrocarbon molecule contains carbon and hydrogen
Q84: Phthalocyanine is a large molecule used in
Q101: A copper ore consisting of 12.5% copper(II)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents