When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 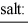
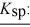 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-5 M Ca2+ + 2 × 10-5 M CO32-
C) 2 × 10-5 M Ca2+ + 2 × 10-3 M F-
D) 2 × 10-2 M Mg2+ + 2 × 10-3 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-
Correct Answer:
Verified
Q51: The solubility product constant of Mg(OH)2 is
Q52: When 100 mL each of 2.0 ×
Q53: When 100 mL each of 2.0 ×
Q54: What is the concentration of free Zn2+
Q55: A homeowner in Boston becomes concerned that
Q57: A solution contains [Ba2+] = 5.0 ×
Q58: To a concentrated buffer of pH 9.0
Q59: In which of the following solutions will
Q60: What is the free Cu2+ concentration if
Q61: Write the solubility product constant expression for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents