Write the equilibrium constant expression for the following reaction: N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A) 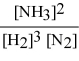
B) 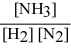
C) 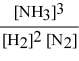
D) 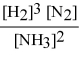
E) 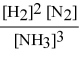
Correct Answer:
Verified
Q43: Equilibrium constant K is constant except when
Q53: Consider the following equation: N2O4(g) ⇌ 2
Q54: What will happen to the equilibrium in
Q55: For the production of NO2, Kc =
Q57: For the reaction; N2(g) + 3 H2(g)
Q59: Consider the following reaction at a certain
Q60: For the reaction below CO(g) + 2H2(g)
Q61: For the reaction: PCl3(g) + Cl2(g) ⇌
Q62: Write the equilibrium constant expression for the
Q63: For the reaction: 2 Cl2(g) + 2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents