What is the equilibrium constant expression for: 4 NH3(g) + 5 O2(g) ⇌ 4 NO(g) + 6 H2O(g)
A) 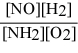
B) 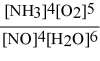
C) 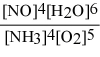
D) 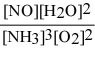
E) 
Correct Answer:
Verified
Q28: In a reaction at equilibrium involving only
Q38: Consider the exothermic reaction: 4 HCl(aq) +
Q39: According to Le Chatelier's Principle:
A) an increase
Q40: Consider the reaction: 2SO2(g) + O2(g) ⇌
Q41: 2.5 moles H2O and 100 g of
Q43: For the decomposition of SO3(g), Kc =
Q44: For the following reaction O2(g) ⇌ 2O(g)
What
Q45: Consider the following equilibrium reaction: PCl3(g) +
Q47: 0.75 mol of N2 and 1.20 mol
Q55: In a reaction at equilibrium involving only
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents