Given the following information, calculate ΔH°(in kcal mol-1) for:
I(g) + e- → I-(g) 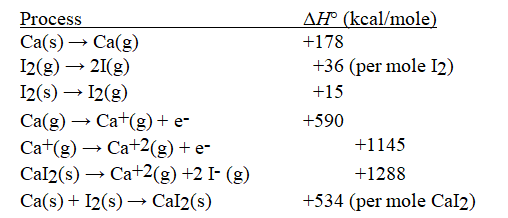
A) -715 kcal mol-1
B) -338 kcal mol-1
C) -89 kcal mol-1
D) -71 kcal mol-1
E) -142 kcal mol-1
Correct Answer:
Verified
Q81: The heat of fusion for napthalene (C10H8)
Q82: All sides are equal in length and
Q83: Which of the following compounds has the
Q85: Ethyl alcohol (CH3CH2OH) has a heat of
Q86: The heat of fusion for water is
Q87: All sides are equal in length and
Q88: The triple point of H2O is at
Q90: Given the following information, calculate ΔH°(in kcal
Q90: The process in which a gas is
Q94: List the following ionic compounds in order
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents