Write a Lewis structure for potassium oxide.
A) 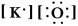
B) K-O
C) K-O-K
D) 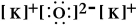
E) 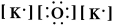
Correct Answer:
Verified
Q75: After drawing the Lewis dot structure for
Q76: What is the molecular shape of BrF5?
A)
Q77: Write a Lewis structure for sodium iodide.
A)
Q78: The electron-pair geometry of H2O is _.
A)
Q79: After drawing the Lewis dot structure for
Q81: Given the listed average bond energies, calculate
Q82: What is the correct molecular geometry for
Q83: Given the bond enthalpies C-O (360), C=O
Q84: Given the bond enthalpies N-H (389), Cl-Cl
Q95: Given the listed bond lengths,what would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents