Determine △H° for the decomposition of hydrogen peroxide. H2O2(l) → 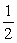 O2(g) + H2O(l)
O2(g) + H2O(l)
Given: H2(g) + O2(g) → H2O2(l) △H° = -187.78 kJ
H2(g) + 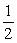 O2(g) → H2O(l) △H° = -285.83 kJ
O2(g) → H2O(l) △H° = -285.83 kJ
A) -98.05 kJ
B) +93.89 kJ
C) -191.94 kJ
D) -93.89 kJ
Correct Answer:
Verified
Q83: Given that ΔH°f [Ag2S(s)] = -32.6 kJ/mole,
Q84: Combine the reactions: P4(s) + 6 Cl2(g)
Q85: How much heat is involved in the
Q86: Using the heat of combustion of methanol
Q87: Given the reactions below, compute ΔH° for
Q89: Determine the enthalpy change in the following
Q90: The heat of combustion, ΔHcomb, of 1-butene(l),
Q91: Compute ΔH°rxn for the following reaction. The
Q92: Given the following thermochemical equations: (kJ)
4 FeS2(s)
Q93: Given the heat of formation of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents