H2S(g) + 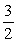 O2(g) → H2O (liq) + SO2(g) ΔH° = -562.3 kJ 1/8 S8(s) + O2(g) → SO(g) ΔH° = -297.0 kJ
O2(g) → H2O (liq) + SO2(g) ΔH° = -562.3 kJ 1/8 S8(s) + O2(g) → SO(g) ΔH° = -297.0 kJ
H2(g) + 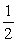 O2 → H2O (liq) ΔH° = -266.9 kJ
O2 → H2O (liq) ΔH° = -266.9 kJ
Compute ΔH° for H2(g) + 1/8 S8 (s) → H2S(g) in kJ.
A) 562.3 kJ
B) 276.6 kJ
C) 265.3 kJ
D) 20.5 kJ
E) -1.6 kJ
Correct Answer:
Verified
Q90: The standard heat of formation of solid
Q91: Compute ΔH°rxn for the following reaction. The
Q92: Given the following thermochemical equations: (kJ)
4 FeS2(s)
Q93: Given the heat of formation of the
Q94: Given that ΔH°f [CO(g)] = -110.5 kJ/mol
Q95: For the reaction H2(g) + 1/2 O2(g)
Q96: Consider the reaction:
CO2(g) + 2HCl(g) →
Q97: Determine the enthalpy change in the following
Q98: Some "beetles" defend themselves by spraying hot
Q100: Determine the enthalpy change in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents