What is the enthalpy of reaction for the neutralization reaction occurring between dilute Ba(OH) 2(aq) and dilute HNO3(aq) ? Values of ΔH°f from data tables are given below. Hint: Balance the equation so the coefficients are the lowest possible set of whole numbers.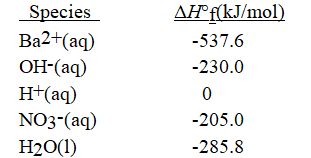
A) +426.0 kJ
B) -111.6 kJ
C) -426.0 kJ
D) -571.6 kJ
E) -460.0 kJ
Correct Answer:
Verified
Q77: If 356 J of work is done
Q78: The complete combustion of propane, C3H8(g), is
Q79: For the reaction H2(g) + 1/2 O2(g)
Q80: What is the work in joules done
Q81: Compute ΔH°rxn for the following reaction. The
Q83: Given that ΔH°f [Ag2S(s)] = -32.6 kJ/mole,
Q84: Combine the reactions: P4(s) + 6 Cl2(g)
Q85: How much heat is involved in the
Q86: Using the heat of combustion of methanol
Q87: Given the reactions below, compute ΔH° for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents