Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 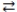 COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
Correct Answer:
Verified
Q70: A reversible reaction in which K =
Q72: When
Q74: Enzymes are proteins that act as biological
Q75: Consider the reaction: 2 C2H6(g)+ 7
Q77: Consider the following reversible reaction at
Q77: Le Châtelier's principle is a general rule
Q78: Consider the following reversible reaction at equilibrium:
Q81: When the equilibrium constant for a reversible
Q81: In the energy diagram shown below, C
Q98: Kinetic energy is the energy associated with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents