Consider the reversible reaction: CO(g)+ Cl2(g) 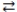 COCl2(g). The reverse reaction is COCl2(g) CO(g)+ Cl2(g).
COCl2(g). The reverse reaction is COCl2(g) CO(g)+ Cl2(g).
Correct Answer:
Verified
Q65: Consider the reaction: 2 C2H6(g)+ 7
Q66: Consider the following reversible reaction at equilibrium:
Q67: A manufacturing company requires 157 kJ
Q70: A reversible reaction in which K =
Q72: When
Q75: Consider the reaction: 2 C2H6(g)+ 7
Q80: The larger the K for a reaction,the
Q81: When the equilibrium constant for a reversible
Q82: A reversible reaction is said to have
Q98: Kinetic energy is the energy associated with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents