Aspartic acid is an amino acid used to synthesize proteins. What is the molar mass of aspartic acid shown below? 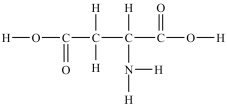
A) 121.09 g/mol
B) 133.11 g/mol
C) 117.0 g/mol
D) 126.04 g/mol
E) 132.09 g/mol
Correct Answer:
Verified
Q4: Write a balanced chemical equation for
Q4: What is the mass of 3.81 mol
Q5: How many moles of carbon dioxide are
Q5: Consider the oxidation of sodium metal
Q8: In the chemical equation 2 Co(NO3)3
Q10: How many moles of sulfur trioxide
Q11: What is the formula weight of KCl?
A)74.55
Q14: Which sample contains the largest number of
Q18: The law of conservation of mass states
Q32: Suppose the theoretical yield in a reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents