Predict the bond angles around the carbon atom in the structure of carbonic acid shown below. Don't forget to draw in lone pairs where needed to give octets. 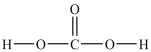
A) 180°
B) 120°
C) 109.5�°
D) 90°
E) 60°
Correct Answer:
Verified
Q1: Which of the statements concerning chemical bonds
Q6: Which bond is the most polar?
A)C-N
B)C-O
C)C-C
D)C-Cl
E)C-F
Q8: How many nonbonded electron pairs are in
Q8: How many covalent bonds are generally formed
Q9: Aspartic acid is an amino acid used
Q10: How many valence electrons are in a
Q12: How many lone pairs of electrons are
Q17: A diatomic molecule contains _. I. atoms
Q21: What are the bond angles in a
Q38: Rank the atoms Br,Cl,and F in order
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents