Using Hess's law and equations 1-3 below, find H° at 25°C for the oxidation of C2H5OH(l) . C2H5OH(l) + 3O2(g) 3H2O(l) + 2CO2(g) 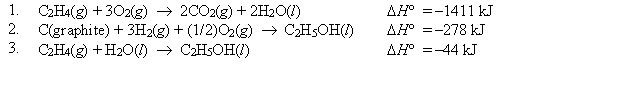
A) -1367 kJ
B) 44 kJ
C) 632 kJ
D) -1742 kJ
E) none of these
Correct Answer:
Verified
Q27: Consider a gas in a 1.0-L bulb
Q36: Consider a process carried out on
Q37: Consider a process carried out on
Q39: Two samples of a monatomic ideal
Q40: Consider a process carried out on 1.00
Q41: A calorimeter contains 95 g of water
Q44: The enthalpy of fusion of ice
Q45: One mole of a liquid is
Q46: Calculate
Q54: A 50.0-g sample of a metal is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents