Given: 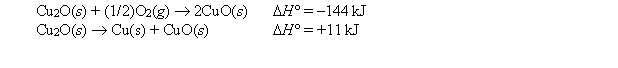 Calculate the standard enthalpy of formation of CuO(s) .
Calculate the standard enthalpy of formation of CuO(s) .
A) +299 kJ
B) +155 kJ
C) -166 kJ
D) -155 kJ
E) -299 kJ
Correct Answer:
Verified
Q48: At 25°C, the following heats of
Q49: A calorimeter contains 143 g of water
Q50: Use the following table: Reaction
Q51: A calorimeter contains 230 g of water
Q52: 75.0 mL of a pure liquid at
Q54: Consider the following numbered processes:
1.
Q55: A 1.00-g sample of the rocket fuel
Q55: The standard enthalpy of formation of
Q56: A 140.0-g sample of water at 25.0°C
Q56: A bomb calorimeter has a heat capacity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents