Choose the correct equation for the standard enthalpy of formation of CO(g) , where H°f for CO = -110.5 kJ/mol.
A) Cgraphite(s) + CO2(g) 2CO(g) , H° = -110.5 kJ
B) Cgraphite(s) + O(g) CO(g) , H° = -110.5 kJ
C) 2Cgraphite(s) + O2(g) 2CO(g) , H° = -110.5 kJ
D) 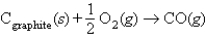 , H° = -110.5 kJ
, H° = -110.5 kJ
E) CO(g) Cgraphite(s) + O(g) , H° = -110.5 kJ
Correct Answer:
Verified
Q70: Consider the following data: 
Q71: The heat of formation of Fe2O3(s)
Q72: Using the information below, calculate
Q73: Which of the following statements is true
Q74: Consider the following reaction:
2Al(s) + 3Cl2(g)
Q74: Standard enthalpies of formation are tabulated on
Q76: The heat combustion of acetylene, C2H2(g),
Q77: For the reaction AgI(s) + (1/2)Br2(g)
Q80: The standard enthalpy change for the
Q85: Consider the following standard heats of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents