Consider the following data: 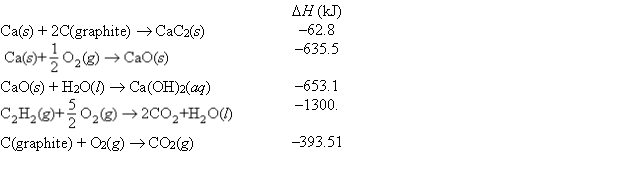 Use Hess's law to find the change in enthalpy at 25°C for the following equation:
Use Hess's law to find the change in enthalpy at 25°C for the following equation:
CaC2(s) + 2H2O(l) C2H2(g) + Ca(OH)2(aq)
Correct Answer:
Verified
Q64: Acetylene (C2H2) and butane (C4H10) are gaseous
Q65: The standard state of carbon as a
Q67: The combustion of methanol takes place
Q69: Using the following data, calculate the
Q71: The heat of formation of Fe2O3(s)
Q72: Using the information below, calculate
Q73: Which of the following statements is true
Q74: Consider the following reaction:
2Al(s) + 3Cl2(g)
Q75: Choose the correct equation for the
Q79: Specific heat capacities are tabulated on a
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents