The standard state of carbon as a free element is graphite. C60 is an allotropic form of carbon belonging to a class of structures known as fullerenes. 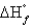 for C60 should be
for C60 should be
A) zero
B) positive
C) negative
D) equal to 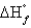 for the other allotropic forms of carbon
for the other allotropic forms of carbon
E) A and D
Correct Answer:
Verified
Q60: When a student performs an endothermic
Q61: For which of the following reaction(s)
Q62: Using the information below, calculate
Q64: Given the following two reactions at
Q64: Acetylene (C2H2) and butane (C4H10) are gaseous
Q67: The combustion of methanol takes place
Q69: Using the following data, calculate the
Q70: Consider the following data: 
Q78: The enthalpy of formation of an element
Q79: Specific heat capacities are tabulated on a
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents