The combustion of methanol takes place according to the reaction 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(l)
Calculate H for the combustion of 1 mol of methanol under standard conditions. Use the following standard enthalpies of formation: 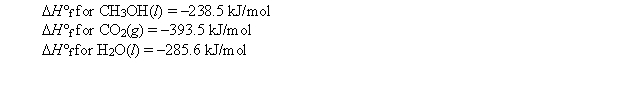
A) -1452.4 kJ/mol
B) +1452.4 kJ/mol
C) -726.2 kJ/mol
D) +726.2 kJ/mol
E) none of these
Correct Answer:
Verified
Q62: Using the information below, calculate
Q64: Acetylene (C2H2) and butane (C4H10) are gaseous
Q64: Given the following two reactions at
Q65: The standard state of carbon as a
Q69: Using the following data, calculate the
Q70: Consider the following data: 
Q71: The heat of formation of Fe2O3(s)
Q72: Using the information below, calculate
Q78: The enthalpy of formation of an element
Q79: Specific heat capacities are tabulated on a
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents