Sodium perbromate can be synthesized via the following reaction that gives off xenon gas. __NaBrO3 + __XeF2 + __H2O 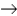 __NaBrO4 + HF + __Xe
__NaBrO4 + HF + __Xe
What stoichiomatric coefficients should be placed in the blanks above to balance the chemical equation?
A) 1, 1, 2, 1, 2, 1
B) 2, 2, 2, 2, 3, 2
C) 3, 2, 1, 3, 2, 1
D) 2, 2, 1, 2, 4, 2
E) None of the above
Correct Answer:
Verified
Q67: The limiting reactant in a reaction
A) is
Q78: Consider the following unbalanced equation: KO2
Q80: Baking powder, a mixture of cream
Q81: Consider the following reaction: 4NH3(g) +
Q82: The characteristic odor of pineapple is due
Q86: Consider the electrolysis of a brine
Q88: Consider the following balanced equation: A(g)
Q95: Hemoglobin is the protein that transports oxygen
Q96: Triphenylene is an organic compound containing only
Q99: When 10.0 g of hydrogen gas reacts
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents