Given the graph below, what is the boiling point of carbon tetrachloride at standard pressure? 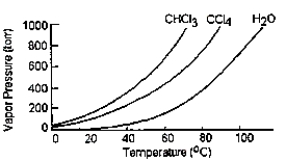
A) The graph does not give this information.
B) 98°C
C) 77°C
D) 60°C
E) 34°C
Correct Answer:
Verified
Q61: The normal boiling point of liquid X
Q65: Which of the following processes must exist
Q67: Which of the following statements is true
Q71: Given below are the temperatures at which
Q72: When 1.00 mol of a pure
Q74: The heat of vaporization of a
Q75: You are given the following boiling-point data:
Q79: How much energy is needed to convert
Q80: Knowing that
Q81: MnO has either a structure like NaCl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents