Choose the correct statement about the diagram below. 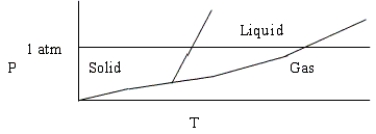
A) The diagram could represent the phase diagram of CO2.
B) The diagram shows the triple point above 1 atm pressure.
C) The diagram is qualitatively correct for water.
D) The diagram shows that the melting point of the solid increases with increasing pressure.
E) None of these statements is correct.
Correct Answer:
Verified
Q64: Make a sketch to show the hydrogen
Q80: Knowing that
Q81: MnO has either a structure like NaCl
Q82: The table below lists the ionic radii
Q86: The table below lists the ionic radii
Q88: The triple point of iodine is at
Q89: Shown below is a phase diagram for
Q90: Consider an ionic compound CxAy where the
Q92: The density of the solid phase of
Q97: 100. g of ice at 0°C is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents