Shown below is a phase diagram for sulfur (not drawn to scale) . Sulfur can exist in solid modifications, rhombic and monoclinic, denoted by SR and SM, respectively. Which of the following statements is incorrect? 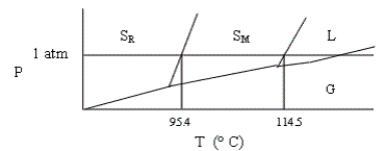
A) At pressures close to 1 atm, rhombic sulfur can be in stable equilibrium with liquid sulfur.
B) Under ordinary atmospheric conditions (at sea level) , sulfur does not sublime.
C) This system has two triple points.
D) At a given pressure, there is (at most) one temperature at which rhombic sulfur can exist in equilibrium with monoclinic sulfur.
E) None of these statements is incorrect.
Correct Answer:
Verified
Q64: Make a sketch to show the hydrogen
Q85: Choose the correct statement about the diagram
Q86: The table below lists the ionic radii
Q90: Consider an ionic compound CxAy where the
Q91: The triple point of CO2 is at
Q92: The density of the solid phase of
Q92: Given the phase diagram shown below, which
Q93: A certain substance has the phase diagram
Q94: Shown below is a phase diagram for
Q97: 100. g of ice at 0°C is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents