Given the phase diagram shown below, which of the following statements is false? 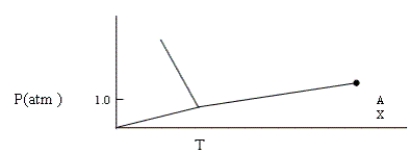
A) When heated at 1 atm, this substance will first melt and then boil.
B) When phase A is compressed at constant temperature at point X, no change is observed.
C) At some (constant) temperature, the gaseous substance can be compressed into a solid and then into a liquid in this order.
D) The solid has a higher density than the liquid.
E) None of these statements is false.
Correct Answer:
Verified
Q64: Make a sketch to show the hydrogen
Q84: KCl crystallizes in a structure like NaCl.
Q89: Shown below is a phase diagram for
Q90: Consider an ionic compound CxAy where the
Q91: The triple point of CO2 is at
Q92: The density of the solid phase of
Q93: A certain substance has the phase diagram
Q94: Shown below is a phase diagram for
Q95: The heat of combustion of bituminous coal
Q97: Below is a phase diagram for compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents