Below is a phase diagram for compound X. You wish to purify a sample of X that was collected at P = 1.0 atm and T = 100 by subliming it. In order to sublime the sample, you should 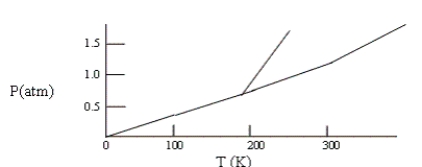
A) increase T to 300 K, keeping P = 1.0 atm.
B) abandon the attempt to sublime X.
C) lower P to 0.5 atm and then increase T to 200 K.
D) increase P to 1.5 atm and then increase T to 300 K.
E) increase T to 300 K and then lower P to 0.5 atm.
Correct Answer:
Verified
Q83: How many grams of ice would be
Q84: KCl crystallizes in a structure like NaCl.
Q86: A certain substance, X, has a triple-point
Q92: Given the phase diagram shown below, which
Q93: A certain substance has the phase diagram
Q94: Shown below is a phase diagram for
Q95: The heat of combustion of bituminous coal
Q100: Based on the phase diagram shown
Q102: Based on intermolecular forces, which organic compound
Q103: Based on intermolecular forces, which of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents