For the reaction 2N2O5(g) 4NO2(g) + O2(g) , the following data were collected. 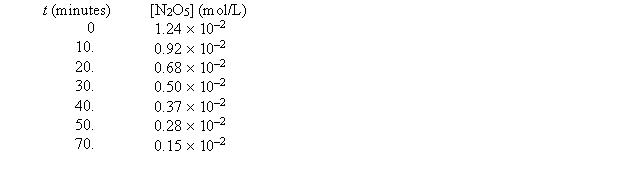 The initial rate of production of NO2 for this reaction is approximately
The initial rate of production of NO2 for this reaction is approximately
A) 6.4 *10-4 mol/L • min
B) 3.2*10-4 mol/L • min
C) 1.24* 10-2 mol/L • min
D) 1.6 * 10-4 mol/L • min
E) none of these
Correct Answer:
Verified
Q1: The following questions refer to the hypothetical
Q6: A general reaction written as 2A +
Q7: The following questions refer to the hypothetical
Q8: A general reaction written as 2A
Q10: A general reaction written as 2A +
Q12: The oxidation of Cr3+ to CrO42- can
Q13: The balanced equation for the reaction
Q14: The rate constant k is dependent on
A)
Q14: A general reaction written as 2A
Q19: The oxidation of Cr3+ to CrO42- can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents