A general reaction written as 2A + 2B C + 2D is studied and yields the following data. 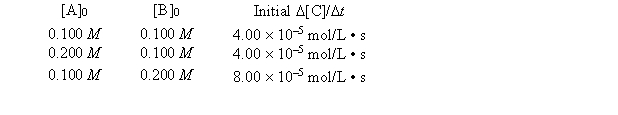
-What is the numerical value of the rate constant?
A) 4.00 * 10-1
B) 4.00* 10-2
C) 4.00 * 10-3
D) 4.00 * 10-4
E) none of these
Correct Answer:
Verified
Q6: A general reaction written as 2A +
Q8: The reaction
H2SeO3(aq) + 6I-(aq) + 4H+(aq) →
Q9: For the reaction 2N2O5(g)
Q9: The oxidation of Cr3+ to CrO42- can
Q10: A general reaction written as 2A +
Q12: The oxidation of Cr3+ to CrO42- can
Q13: The balanced equation for the reaction
Q17: The average rate of disappearance of
Q18: A first-order reaction is 54% complete at
Q19: For the reaction
2A + B
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents