Consider the following Lewis structure. (Lone pairs are not drawn in.) 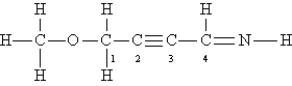 What are the hybridizations of the oxygen atom and of carbon atoms 1, 2, and 4, respectively (order: O C-1 C-2 C-4) ?
What are the hybridizations of the oxygen atom and of carbon atoms 1, 2, and 4, respectively (order: O C-1 C-2 C-4) ?
A) sp2 sp3 sp2 sp3
B) sp sp3 sp sp
C) sp3 sp3 sp sp2
D) sp sp2 sp sp2
E) sp sp3 sp2 sp
Correct Answer:
Verified
Q22: Tetracyanoethylene has the skeleton shown here:
Q27: Consider the following molecule. (Lone pairs are
Q35: Tetracyanoethylene has the skeleton shown here:
Q37: Specify the hybridization of the nitrogen atom
Q38: Which of the following has two
Q42: Which of the following is diamagnetic?
A) O2-
B)
Q43: Which of the following species is paramagnetic?
A)
Q45: Which of the following statements is false?
A)
Q57: Order the following from shortest to longest
Q58: The fact that O2 is paramagnetic can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents