Calculate E at 25°C for this cell, given the following data: 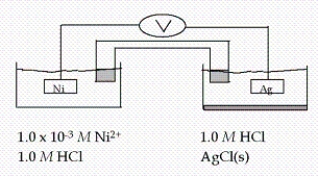 Ag+ + e- → Ag(s) E° = 0.80 V
Ag+ + e- → Ag(s) E° = 0.80 V
Ni2+ + 2e- → Ni(s) E° = -0.23 V
Ksp for AgCl = 1.6 × 10-10
A) 2.98 V
B) This cannot be determined from the data given
C) 0.83 V
D) 1.01 V
E) 0.54 V
Correct Answer:
Verified
Q57: A galvanic cell is constructed with copper
Q58: A concentration cell is constructed using two
Q59: Refer to the galvanic cell below (the
Q60: You make a cell with a copper
Q61: In a common car battery, six identical
Q63: Fe2+ + 2e- → Fe(s) E° =
Q64: An antique automobile bumper is to be
Q65: A solution of MnO42- is electrolytically reduced
Q66: If an electrolysis plant operates its electrolytic
Q67: How many seconds would it take to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents