Which of the following would be a good sacrificial electrode to protect an iron boat from corrosion? 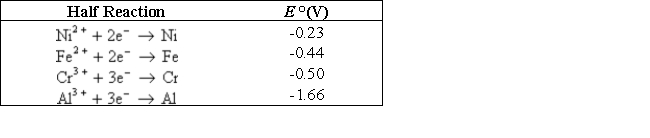
A) Nickel
B) Chromium
C) Aluminum
D) B and C
E) More information needed to answer the question
Correct Answer:
Verified
Q80: Gold is produced electrochemically from an aqueous
Q81: Which of the following statements is/are true
Q82: Since aluminum is a very active metal,
Q83: Which of the following statements is/are true
Q84: When a battery dies, which of the
Q85: Cu2+ + 2e- → Cu(s) E° =
Q86: The SI unit for current is the
Q87: In the following equation
ΔG = ΔG° +
Q88: Which of the following statements is/are true
Q89: Consider the given cathodic reaction.4Fe2+(aq) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents