Given the following data, calculate the normal boiling point for formic acid (HCOOH) . 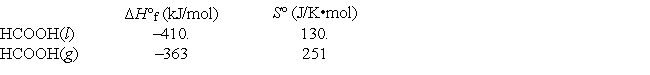
A) 115°C
B) 82°C
C) 2.57 K
D) 1730°C
E) 388°C
Correct Answer:
Verified
Q75: As O2(l) is cooled at 1 atm,
Q76: Elemental sulfur exists in two crystalline forms,
Q77: For the vaporization of a liquid at
Q78: For the reaction A + B →
Q79: At constant pressure, the reaction
2NO2(g) → N2O4(g)
Is
Q81: The standard free energy of formation of
Q82: Assume that the reaction
CO(g) + H2O(g)
Q83: The reaction is allowed to proceed until
Q84: Consider the gas phase reaction
NO + (1/2)O2
Q85: Consider the reaction
2N2O5(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents