Elemental sulfur exists in two crystalline forms, rhombic and monoclinic. From the following data, calculate the temperature at which monoclinic sulfur and rhombic sulfur are in equilibrium. 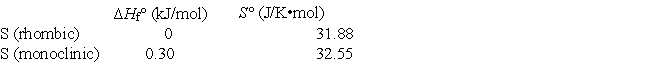
A) 0 K
B) +450 K
C) +210 K
D) -210 K
E) -450 K
Correct Answer:
Verified
Q71: For the process involving compound A: A(s)
Q72: For a spontaneous endothermic process, which conditions
Q73: Substance X has a heat of vaporization
Q74: Calculate ΔG for the vaporization of Br2(l)
Q75: As O2(l) is cooled at 1 atm,
Q77: For the vaporization of a liquid at
Q78: For the reaction A + B →
Q79: At constant pressure, the reaction
2NO2(g) → N2O4(g)
Is
Q80: Given the following data, calculate the normal
Q81: The standard free energy of formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents