A certain chemical reaction sets up an equilibrium as follows
3A2(aq) + 2B3(aq) 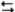 6AB(aq)
6AB(aq)
After being started with unknown amounts of A2 and B3. Once the system reaches equilibrium the concentrations of A2, B3, and AB are precisely determined and the temperature recorded. What other quantities can be determined? Q 0 is the reaction quotient at the initial conditions.
A) Q 0, Kc, K, ΔG
B) Q 0, K, ΔG, ΔG°
C) K, Kc, ΔG, ΔG°
D) Q 0, Kc, ΔG, ΔG°
E) Q 0, Kc, K, ΔG°
Correct Answer:
Verified
Q133: In which of the following changes is
Q134: A gas phase decomposition reaction
2AB3 
Q135: Identify the second law of thermodynamics.
A) In
Q136: For a certain process, at 311 K,
Q137: For a certain process, at 319 K,
Q138: For the process A(l) → A(g), ΔH°
Q139: One mole of an ideal gas is
Q141: Consider 8.0 moles of a monatomic ideal
Q142: A sample contains 12.0 moles of a
Q143: Which of the following statements is/are true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents