At 25°C, the following heats of reaction are known: 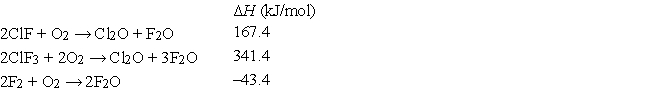 At the same temperature, calculate ΔH for the following reaction:
At the same temperature, calculate ΔH for the following reaction: 
A) -217.5 kJ/mol
B) -108.7 kJ/mol
C) +217.5 kJ/mol
D) -130.2 kJ/mol
E) none of these
Correct Answer:
Verified
Q41: A calorimeter contains 95 g of water
Q42: A bomb calorimeter has a heat capacity
Q43: Use the following table: Q44: Using Hess's law and equations 1-3 below, Q45: When a student performs an endothermic reaction Q47: One mole of a liquid is vaporized Q48: One mole of a liquid is vaporized Q49: Calculate ΔH° for the reaction C4H4(g) + Q50: The standard enthalpy of formation of H2O(l) Q51: A calorimeter contains 240 g of water![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents