Choose the correct equation for the standard enthalpy of formation of CO(g) , where ΔH°f for CO = -110.5 kJ/mol.
A) Cgraphite(s) + CO2(g) → 2CO(g) , ΔH° = -110.5 kJ
B) Cgraphite(s) + O(g) → CO(g) , ΔH° = -110.5 kJ
C) 2Cgraphite(s) + O2(g) → 2CO(g) , ΔH° = -110.5 kJ
D) 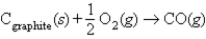 , ΔH° = -110.5 kJ
, ΔH° = -110.5 kJ
E) CO(g) → Cgraphite(s) + O(g) , ΔH° = -110.5 kJ
Correct Answer:
Verified
Q75: For the reaction
AgI(s) + (1/2)Br2(g) → AgBr(s)
Q76: Given the following two reactions at 298
Q77: Using the information below, calculate ΔH°f for
Q78: The enthalpy of formation of an element
Q79: Specific heat capacities are tabulated on a
A)
Q81: In the _ process, the heavier molecules
Q82: The most common method of producing _
Q83: Compounds A and B have the same
Q84: Which of the following statements is/are true
Q85: Identify the potential fuel that can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents